Dengue Hemorrhagic Fever, Copepods, and Biological Control of Mosquitoes in Vietnam
Gerald G. Marten
J. Policy Studies (Japan) 9: 131-141. 2000 - Download .pdf (860kb)
The story of Dengue Hemorrhagic Fever, an “emergent” disease known only since 1950, illustrates the benefits that are possible with local community action and ecological management. Dengue Hemorrhagic Fever is transmitted by mosquitoes that breed in water storage tanks and other water-filled containers around people’s homes. There is no vaccine or medicine for this virus; the only way to prevent the disease is to get rid of the mosquitoes. Initially spectacular success controlling the mosquitoes with DDT during the 1950s and 1960s was not sustainable because the mosquitoes evolved resistance to DDT. Other pesticides have gone into use, but there has been no overall reduction in the disease. Nearly one hundred million people in tropical Asia and Latin America are infected with dengue each year, about one-half million children are afflicted with life-threatening Dengue Hemorrhagic Fever, and thousands die. The pesticide-based strategy of the last fifty years has been a failure, but what else can be done? Ecological management promises to be more effective and sustainable. The copepod Mesocyclops provides an example. This tiny crustacean kills virtually all the mosquito larvae whenever it is in water-filled containers where mosquitoes breed. Vietnam has mounted a campaign to distribute Mesocyclops throughout the country. The mosquito and the disease have disappeared from every village that uses Mesocyclops, the key to success being strong community organization to ensure that everyone uses the copepods.
Introduction
It was a significant moment for the control of mosquito-transmitted diseases when in August 1994 the mosquito Aedes aegypti disappeared from Phanboi, a village in northernVietnam. Aedes aegypti is the principal vector of Dengue Hemorrhagic Fever (Halstead 1997, 1998), a disease unknown before 1950. This “emergent” disease has hospitalized nearly two million Vietnamese and killed more than 13,000 Vietnamese children since appearing in Vietnam forty years ago. Phanboi has been free of Aedes aegypti and the disease for six years, and numerous other communities in Vietnam are achieving the same. The keystone to success is biological control with Mesocyclops, a tiny crustacean that preys on mosquito larvae. The story of Dengue Hemorrhagic Fever and Mesocyclops illustrates how modernization creates new public health problems and how ecological technologies and ecological management can contribute to sustainable solutions.
Dengue is a flavivirus related to yellow fever. It may have originated in non-human primates, which still provide a natural reservoir in Africa and Asia. Non-human primates do not show symptoms, but humans can be seriously ill. First-time dengue infections in children are usually mild, often unnoticed, but first-time infections in adults are often severe. Fatalities are rare, but high fever, chills, headache, vomiting, severe prostration, muscle and bone aches, and severe weakness for more than a month after the fever subsides make dengue fever an illness that many adults remember as the worst they ever experienced.
Dengue Hemorrhagic Fever is a life-threatening form of dengue. It is not caused by a separate viral strain. It comes from the fact that the dengue virus has four serotypes. Infection with one serotype confers lifelong immunity to that serotype while also creating antibodies that enhance infection with other serotypes. Dengue Hemorrhagic Fever typically occurs when infection with one serotype is followed a year or more later by infection with another serotype. About 3% of second infections produce Dengue Hemorrhagic Fever, and about 40% of Dengue Hemorrhagic Fever cases develop a shock syndrome that can be fatal. The most serious consequence of Dengue Hemorrhagic Fever is fluid leakage from capillaries into tissues and body cavities, sometimes accompanied by severe gastrointestinal bleeding (hence the name “hemorrhagic”). There is no medicine to counter the virus, but the loss of fluids can be treated by getting water and electrolytes into the vascular system, administered orally in mild cases and intravenously in severe cases. Most Dengue Hemorrhagic Fever victims are under 15 years old. If untreated, about 5% of Dengue Hemorrhagic Fever cases are fatal, but proper treatment can reduce fatalities to less than 1%.
Aedes aegypti (Christophers 1960) is the principal vector of both dengue and yellow fever. Originally a tree-hole-breeding mosquito in Africa, it long ago acquired an urban lifestyle by breeding in similar situations around human habitations. Aedes aegypti now breeds in man-made containers such as water storage tanks, wells, clogged rain gutters, and discarded objects such as tires, tin cans, and jars that collect rainwater. The mosquito lays her eggs on the side of a container a few millimeters above the water level. The eggs can sit for months without hatching if they remain dry, but they hatch within minutes if covered with water. The fact that this normally happens only when more water is added to a container increases the probability that a container will have enough water for the larvae to complete their development before the container dries out.
While male mosquitoes feed only on plant juices, females suck blood from animals to get nutrients they need to develop their eggs. When a female takes blood from a person infected with dengue, the virus multiplies in her body, and 7-15 days later (depending on temperature) she has enough of the virus to infect people. Transmission of the virus is much higher in tropical climates where rapid viral multiplication at higher temperatures makes it more likely for an infected mosquito to survive long enough to become infectious.
History of Dengue
Starting in the Sixteenth Century with expansion of European colonialism and trade, Aedes aegypti spread around the world by hitching rides in water storage containers on boats. Dengue and Aedes aegypti were together in Asia for centuries without a serious problem because the distribution of Aedes aegypti was limited by Aedes albopictus, an indigenous Asian mosquito that is physiologically capable of transmitting dengue but not associated with significant dengue transmission in practice. Asian towns and cities were well endowed with trees and shrubs, and Aedes albopictus competitively excluded Aedes aegypti wherever there was vegetation.
The situation in the Americas was quite different. Aedes aegypti thrived in cities and towns because no mosquito like Aedes albopictus restricted its distribution. We know Aedes aegypti was common in the Americas because of numerous yellow fever epidemics following the introduction of yellow fever from Africa by the slave trade in the Sixteenth Century. The historical record for dengue is not clear because its symptoms do not distinguish it clearly from other diseases, but dengue was probably common through much of the Americas for centuries. Philadelphia had a dengue fever epidemic in 1780. Dengue fever was common in towns and cities on the Gulf and Atlantic coasts of the United States until the 1930s.
Dengue probably spread everywhere with Aedes aegypti, but it did not attract much attention where the infection rate was high, because most people were infected as children with mild symptoms. Devastating epidemics made yellow fever a very different matter. Aedes aegypti became an object of international attention when Walter Reed demonstrated in 1900 that this mosquito was responsible for yellow fever transmission. Campaigns were initiated in the Americas to get rid of Aedes aegypti by eliminating the places where it was breeding around people’s houses. During the 1930s the Rockefeller Foundation mobilized a virtual army of house-to-house government inspectors in Brazil to find and eliminate every place Aedes aegypti might breed. Inspectors had legal authority to enter premises, destroy containers, apply oil or paris green (an arsenic mosquito larvicide), and impose fines. It was possible to consolidate the eradication of Aedes aegypti neighborhood by neighborhood, without reinvasion, because adult Aedes aegypti usually travels less than 100 meters in a lifetime. With meticulous quality control, the campaign was so effective that Aedes aegypti was eradicated from large areas of Brazil by the early 1940s (Soper et al. 1943).
Although there were outbreaks resembling Dengue Hemorrhagic Fever in Queensland, Australia in 1897 and in Greece in 1928, Dengue Hemorrhagic Fever was not a recognized disease until 1956 because it was unusual to have more than one serotype in the same place. Everything changed with World War II, when large numbers of people and the four dengue serotypes were moved around the Asian tropics. There were numerous dengue fever epidemics during the war as the virus was introduced to new areas where people lacked immunity. Cases with Dengue Hemorrhagic Fever symptoms appeared in Thailand in 1950. The first recognized Dengue Hemorrhagic Fever epidemic was in the Philippines in 1956, followed by epidemics in Thailand and other parts of Southeast Asia within a few years. Uncontrolled growth of Third World cities during the following decades greatly expanded Aedes aegypti’s breeding habitat. Urban landscapes provided a bounty of water storage tanks and discarded containers collecting rainwater in neighborhoods lacking basic services such as piped water and trash collection. The decline of vegetation in urban landscapes allowed Aedes aegypti to expand through Asian cities without competition from mosquitoes like Aedes albopictus.
The spread of Dengue Hemorrhagic Fever was probably delayed by the appearance of DDT in 1943. DDT was like a miracle. It was harmless to vertebrates at concentrations used to kill insects, and it was effective for months after application. In 1955 the World Health Organization began a global campaign to spray every house in malarial areas with DDT. Malaria virtually disappeared from many areas by the mid-1960s, and at the same time Aedes aegypti disappeared from most of Latin America and some parts of Asia such as Taiwan.
The incredible success of DDT was short-lived because mosquitoes evolved resistance that spread quickly around the world, probably accelerated by heavy use of DDT for agriculture. Third World governments could not afford to continue intensive spraying, particularly when alternatives to DDT such as malathion cost more than ten times as much. Malaria started to return in force by the late 1960s, and by the mid-1970s Aedes aegypti returned to the areas from which it had previously been eradicated. Dengue did not return to the United States because window screening and air conditioning led to an indoor life style that reduced the contact between people and mosquitoes. However, the four dengue serotypes and Dengue Hemorrhagic Fever spread rapidly through tropical Asia, settling into a permanent pattern of recurring local Dengue Hemorrhagic Fever outbreaks as the four serotypes continued to circulate. Dengue Hemorrhagic Fever entered the Americas in 1981 with an epidemic in Cuba that hospitalized 116,000 people in three months. Dengue quickly spread through much of Latin America, sometimes punctuated by dengue fever epidemics of hundreds of thousands of people, but Dengue Hemorrhagic Fever was generally sporadic because most areas had only one serotype. Although dengue was common in many parts of sub-Sahara Africa, it was not a major health problem because Africans are generally resistant to severe dengue infection.
The social and political situation for dealing with Aedes aegypti had changed immensely since the campaigns against yellow fever earlier in the century. A few wealthier countries such as Taiwan continued to spray houses with newer insecticides, and a few countries such as Cuba and Singapore initiated comprehensive house inspections and fines to get rid of Aedes aegypti breeding around people’s homes. However, most countries lacked the political will and the financial and organizational resources to implement such programs. Chemical larvicides, and later a microbial larvicide (Bacillus thuringiensis), were available to treat water storage containers, but people were reluctant to put pesticides in their water. Even if people are willing, larvicides must be applied continuously every 1-3 weeks (depending on the larvicide) to be effective. The cost of purchasing larvicides and managing large-scale use proved beyond the capacity of every government that tried to do it. Some governments tried to organize voluntary community participation to eliminate Aedes aegypti breeding habitats – advising housewives, for example, to clean their water storage containers weekly to interrupt development of the larvae – but without much success.
Today the main action against mosquitoes is by individual families who purchase insecticide spray cans and mosquito coils to keep mosquitoes from bothering them at night. The effect on Aedes aegypti is limited because this mosquito bites during the day and spends most of its time resting in places like clothes closets beyond the reach of casual spraying. Vaccine development has been under way for years, but progress has been slow, and a vaccine could be risky because it might enhance susceptibility to Dengue Hemorrhagic Fever as happens after natural dengue infections. It is now typical in most places for governments to do little about Aedes aegypti until there is a dengue epidemic or Dengue Hemorrhagic Fever appears. Then trucks drive up and down the streets spraying malathion, with little impact in many instances because the epidemic is already well under way and female Aedes aegypti are inside houses where not much insecticide can reach them. To sustain any impact, spraying must be frequent because Aedes aegypti can rebound to large numbers within a few days.
There has been no noticeable decrease in dengue fever or Dengue Hemorrhagic Fever cases during the past 20 years. Worldwide about 50-100 million people are infected with dengue each year. There are several million clinical dengue fever cases and 400,000-500,000 Dengue Hemorrhagic Fever cases each year. Fatalities have remained high in some countries, but other countries have reduced fatalities dramatically by providing extensive medical treatment. Several hundred thousand people are hospitalized with Dengue Hemorrhagic Fever in Vietnam and Thailand every year, but the fatality rate is less than 0.3%. Nonetheless, the economic costs are high. Patients require 1-3 weeks of hospitalization, and parents lose work time while caring for sick children in hospitals. Global warming could eventually extend the geographic range of dengue as higher temperatures, and consequently shorter viral incubation times in mosquitoes, stimulate transmission.
Copepods Enter the Scene
Though biological control with predators of Aedes aegypti larvae offers the possibility of functioning without the frequently repeated applications necessary for pesticides, biological control did not receive serious consideration when the DDT strategy collapsed. Fish were widely used against malarial mosquito larvae prior to the DDT era, but the use of fish for Aedes aegypti control was limited because fish were expensive and did not survive for long in most containers. Besides, many people did not want fish in their water storage containers, particularly if they used the water for drinking. Many aquatic animals such as planaria, dragon fly nymphs, and aquatic bugs were known to prey on mosquito larvae, but none had ever proved effective enough or practical enough to go into operational use. Mosquito-control professionals and public health officials, who relied heavily on chemical pesticides throughout their careers, considered biological control a “pipe dream”. Opportunities for profit were too remote to stimulate research and development by the private sector.
This was the situation in the early 1980's, when Francois Riviere (“Louis Malarde” Medical Research Institute, Tahiti) reported that virtually no Aedes larvae survived in water-filled containers if the copepod Mesocyclops aspericornis was present (Riviere and Thirel 1981). Soon after, the same thing was observed independently in Colombia and Hawaii (Suarez et al. 1984, Marten 1984).
Cyclopoid copepods are different from other aquatic invertebrates that prey on mosquito larvae. If mosquito larvae are numerous, cyclopoids eat only a small part of each larva, giving each copepod the capacity to kill 30-40 larvae per day, far more than they actually eat. Even more important is their large numbers. Cyclopoids eat small animals up to twice their own size, but they also eat phytoplankton, protozoa, and rotifers – a diet that provides enough food to make cyclopoid copepods the most abundant predator in most freshwater habitats. The total capacity of a cyclopoid population to kill mosquito larvae is enormous. Most species of cyclopoids are too small (0.3-1.2 mm body length) to prey on even the smallest mosquito larvae, but Mesocyclops aspericornis and other large species of cyclopoids (1.2 mm body length or more) attack and consume newly hatched mosquito larvae without hesitation. About 10% of the places with water where mosquitoes might breed have natural populations of Mesocyclops or other large cyclopoids, which drastically reduce the survival of mosquito larvae. Malarial mosquito larvae (Anopheles) have been observed to be consistently absent from aquatic habitats in Latin America that contained natural populations of Mesocyclops longisetus, the largest known species of Mesocyclops (Marten et al. 1989).
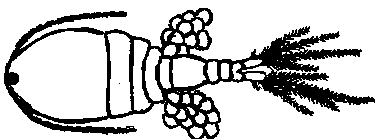
Figure 1. Mesoscyclops. The actual body length is about 1.5 millimeters. Copepods do not have eyes. The eyespot in the middle of the forehead detects light but does not form an image. Copepods move by means of rapid oar-like movements of their large antennules (the long structures extending to each side of the body from the front). The antennules contain mechanical sensory organs that detect vibrations in the water so copepods know when small animals such as mosquito larvae are close enough to be captured as food. Female copepods carry egg sacs on both sides of their body for about three days until young copepods emerge from the eggs. (Click View/Page Layout in Microsoft Word to see this figure.)
The same thing that happens in nature can be achieved by introducing appropriate cyclopoid species to sites that don’t already have them. Anopheles larvae virtually disappeared after Mesocyclops longisetus and several other species of Mesocyclops were introduced to rice fields and small marsh areas in Louisiana (Marten et al. 1994a). Unfortunately, the potential of cyclopoids for malaria control has not been developed further because control of Anopheles mosquitoes has been almost entirely abandoned. Ecological management of relatively complex Anopheles breeding habitats has not appealed to malaria-control practitioners accustomed to the simplicity of pesticides. Contemporary malaria control is based almost entirely on anti-malarial drugs, whose long-term effectiveness is doubtful due to drug-resistance already widespread among malarial parasites.
The development of cyclopoid copepods for dengue control has been much more successful because cyclopoids are so effective and easy to use in the simple container habitats where Aedes aegypti breeds. It is unusual for cyclopoids to get into man-made containers on their own, but they thrive in many kinds of containers when introduced, and they do so independently of the supply of mosquito larvae. Typical populations are 100 adult copepods in a rainwater-filled tire, 500-2,000 in a 200-liter water storage drum, and 10,000 in a larger cement tank. The largest species usually kill more than 99% of the Aedes aegypti larvae, and they usually stay in a container for as long as there is water. Even without water, they can survive as long as there is moisture.
Francois Riviere conducted the first large-scale field trials by collecting natural Mesocyclops aspericornis populations from aquaculture ponds and introducing them to several thousand crab holes that provided breeding habitat for a native species of Aedes mosquito in Tahiti (Riviere et al. 1987). The field trials confirmed the devastating effect that Mesocyclops could have on mosquito larvae, but there was little impact on the mosquito population as a whole. The copepods were effective as long as there was water in a crab hole, but they disappeared when a hole dried out, and mosquitoes returned as soon as the hole had water again.
Subsequent research focused on finding the best copepod species to use against Aedes aegypti in various parts of the world. Suitable species were always available locally because cyclopoids large enough to kill mosquito larvae occur naturally virtually everywhere that Aedes aegypti is a problem. Brian Kay (Queensland Institute of Medical Research) surveyed Australian and Southeast Asia cyclopoids and field-tested them in wells and water storage containers (Brown et al. 1991, Jennings et al. 1995, Russell et al. 1996). The New Orleans Mosquito Control Board began a search for promising species in the Western Hemisphere, developing procedures to mass-produce copepods for operational use and working out details of how to use them (Marten 1990a, Marten et al. 1994a, 1994b).
The simple life cycle of cyclopoid copepods and their ability to thrive on a diet of protozoa made mass production easy and inexpensive. The New Orleans Mosquito Control Board’s production system (Marten et al. 1997) uses bacteria on decomposing wheat seed as food for Chilomonas (a flagellate protozoan that provides food for young cyclopoids) and Paramecium caudatum (a ciliate protozoan that provides food for the larger stages). The system is simple, inexpensive, and highly resilient, functioning in open containers of any size or shape. One hundred adult female Mesocyclops produce about 25,000 new adult females within a month. Females are inseminated during adolescence and require no further contact with males to produce 50-100 eggs weekly during their several month life span. Although males and females are born in equal numbers, adult females enormously outnumber adult males because females are larger and eat the males.
Taxonomic revisions and species identifications by copepod specialists have been crucial for evaluating the potential of copepods for practical mosquito control because superficially similar species can be so different in their ability to survive in different aquatic habitats. Janet Reid (Smithsonian Institution) has had a central role in almost every study during the past fifteen years. More recently Maria Holynska (Zoological Institute, Polish Academy of Sciences) revised Southeast Asian Mesocyclops and assisted with their identification.
The New Orleans Mosquito Control Board found that that nearly all large cyclopoid copepods prey on mosquito larvae, except gigantic Homocyclops ater (3 mm body length), which has completely different habits from other cyclopoids. However, predation on Aedes aegypti larvae was not in itself sufficient for effective Aedes aegypti control. Natural populations of Acanthocyclops vernalis, for example, killed mosquito larvae in temporary pools on the ground, where this copepod could survive in dry soil for a more than a year. When introduced to containers, Acanthocyclops eliminated Aedes aegypti larvae if the adult copepods grew to full size, but Acanthocyclops often multiplied to such large numbers in containers that they exhausted their food supply, making them too stunted to kill Aedes aegypti larvae.
In 1989 the introduction of Mesocyclops longisetus and Macrocycops albidus to rain-filled tires in wooded areas of New Orleans provided the first impressive demonstration of what cyclopoids could do to entire mosquito populations (Marten 1990b). Discarded tires were a major source of three species of Aedes mosquitoes in New Orleans: Aedes aegypti, Aedes albopictus, and Aedes triseriatus. The Aedes mosquito populations completely collapsed around every treated tire pile. The copepods remained in more than 90% of the treated tires through five subsequent years of observation, and the mosquito populations did not reappear. Subsequent field trials determined that the most effective immediate and long-term control of Aedes larvae could be achieved with a single application of cyclopoids to tires or other Aedes breeding containers in combination with the microbial larvicide BTI (Bacillus thuringiensis) (Marten et al. 1993). Copepods could be introduced into thousands of discarded tires in a matter of minutes, using a forced-air insecticide sprayer that blasted the copepods over a tire pile without harming them.
Macrocyclops albidus is a temperate species of potential use in colder climates, where it can survive zero-degree water temperatures. It also lives in the tropics but is not suitable for use in Aedes aegypti breeding containers in the tropics because it is killed by water temperatures above 37º C. The New Orleans Mosquito Control Board survey concluded that Mesocyclops longisetus, which has a natural distribution from southeastern United States to Argentina, is the best species for Aedes aegypti control in the Western Hemisphere. Mesocyclops longisetus survives water temperatures up to 43º C in sun-exposed containers, and because it clings to the bottom and sides of a container, it survives in water storage containers from which people frequently scoop water. Mesocyclops species that swim in the water column quickly disappear from a water storage container.
Financial support from the Rockefeller Foundation and USAID’s Vector Biology and Control Project provided the opportunity to conduct field trials with Mesocyclops longisetus in Honduras and Puerto Rico (Marten et al. 1992, Marten et al. 1994b). Mesocyclops longisetus was particularly effective in wells, cisterns, 200-liter drums, clay jars, flower vases, and bromeliads that had water on a continuous basis. It did not survive in small rainwater-filled containers or discarded tires that dried out frequently, though it did well in tires that were continuously filled with water during the rainy season. It did not survive in small cement tanks with rapid water turnover, particularly if the water was frequently run down the drain, and it was killed when bleach was left in a tank after cleaning or slopped into a tank while washing clothes next to the tank.
People did not object to copepods in their water storage containers because it was not unusual to have small aquatic animals in the water. Swallowing copepods was seldom an issue because the water in these containers was generally not used for drinking. Besides, most Mesocyclops longisetus stay in a container when water is removed for use, and they are harmless if swallowed. People could filter the water through a cloth if they wanted to be sure there were no copepods.
The main difficulty was the loss of copepods from water storage containers when they were cleaned. This was overcome by saving a small quantity of water from the container to restock it with Mesocyclops after cleaning. Though cyclopoids are very small, their unique body shape and jerky motion made it easy for people to confirm their presence in a glass of water removed from the bottom of a container. Housewives in small-scale neighborhood projects in Honduras (Marten et al. 1994b) and Brazil (Vasconcelos et al. 1992) quickly learned to monitor their containers and maintained Mesocyclops at their homes with pride, the key to success being personal attention from community organizers. Unfortunately, Latin American public health bureaucracies lacked the capacity for neighborhood organization to expand the use of Mesocyclops to a larger scale.
Success in Vietnam
The first demonstration of how effective Mesocyclops could be on a larger scale began in 1993, when Vu Sinh Nam (Vietnam National Institute of Hygiene and Epidemiology) introduced three local species of Mesocyclops (Mesocyclops woutersi, Mesocyclops thermocyclopoides, Mesocyclops ruttneri) to Phanboi. Like most of rural Vietnam, the two main sources of Aedes aegypti in this village of 400 houses were several-thousand-liter cement tanks that nearly every house uses for long-term storage of rainwater from the roof, and clay jars (20-200 liter capacity) used to store water for immediate use. Vietnam had tried projects with fish for people to put in their containers, but long-term coverage of the important containers in a community seldom exceeded 20%.
Mesocyclops was much more successful (Nam et al. 1998). All three Mesocyclops species thrived in the large cement tanks, which are seldom drained or cleaned. They did nearly as well in large clay jars but could not survive for long in small clay jars because the water was frequently poured out. Introduction of Mesocyclops to wells provided a reservoir that continually restocked clay jars used to store well water.
Over the course of a year the Aedes aegypti population in Phanboi declined to about 5% of the population in a nearby control village. However, Aedes aegypti was still breeding in small discarded containers like jars, bottles, and cans that collected rainwater but could not be treated with Mesocyclops. The scientists had not actively involved the community before this because they wanted to confirm how well Mesocyclops worked before raising people's expectations. Villagers were now encouraged to participate. Motivation was high due to a history of Dengue Hemorrhagic Fever outbreaks, and the socialist political system provided a basis for rapid, comprehensive, and continuous community mobilization. The village Women’s Union educated villagers about the use of Mesocyclops and organized villagers to stock any containers without Mesocyclops by pouring in a small quantity of water from containers that already had them. An existing recycling program for discarded containers was reorganized to ensure they did not collect rainwater while waiting for pickup.
Aedes aegypti disappeared completely within another five months. No adult or larval Aedes aegypti has been seen in Phanboi during the past six years, though Aedes aegypti continued to be abundant in all other villages in the district. Mesocyclops was then introduced to the rest of the villages in the same commune and disappeared from them as well. The disappearance of Aedes aegypti was of international significance because it was the first time in more than 20 years that even local eradication of any kind of mosquito had been documented anywhere in the world, and it was accomplished without pesticides.
It is significant that Aedes aegypti disappeared without having Mesocyclops in every container. Success was probably due to the “egg trap effect”. Egg-laying mosquitoes do not discriminate against containers with Mesocyclops, so they waste their eggs on containers with Mesocyclops instead of putting them in containers with better prospects for larval survival. Simulation studies with the Container Mosquito Simulation Model developed by Dana Focks (Focks et al. 1993) indicate that a mosquito population will collapse if Mesocyclops is in more than 90% of the containers. In contrast, getting rid of 90% of the containers only reduces mosquito populations in the model by 90%.
The successful demonstration at Phanboi was essential for mobilizing official government support and foreign financial assistance to distribute Mesocyclops to more communities in Vietnam. The National Institute of Epidemiology and Hygiene developed procedures for distributing Mesocyclops on a larger scale with financial support from the Australian Foundation of Peoples of Asia and the Pacific and the Netherlands-Vietnam Medical Commission and technical collaboration from Brian Kay.
A simple “intermediate technology” mass production system at the National Institute of Hygiene and Epidemiology uses a hundred 150-liter plastic garbage pails to produce 200,000 Mesocyclops per month at very low cost. Four native Vietnamese species are produced: Mesocyclops woutersi, Mesocyclops ruttneri, Mesocyclops thermocyclopoides, and Mesocyclops aspericornis. A community receives the species that is naturally most common in containers of its region. The program has trained about 800 health workers and collaborators, and Mesocyclops has been distributed to 30,000 households in northern and central Vietnam.
The program follows the Phanboi model. Central staff train local health workers, who in turn use videotape shows to introduce Mesocyclops to the community. The health workers train local teachers to organize students for regular collection of discarded containers. From the village women's union, health workers recruit volunteer "collaborators" with demonstrated reliability in ongoing house-to-house family planning and immunization programs. Each collaborator is paid $2-$4/month and is responsible for 50-100 houses. Collaborators start by introducing about 50 Mesocyclops into a tank at one of the houses. As soon as the copepods multiply to large numbers, a collaborator carries a bucket of tank water containing Mesocyclops around to all the other houses, pouring a glass of the water into every container. Collaborators explain the use of Mesocyclops to every family and return at least once a month to inspect the containers and update their records. They periodically visit each other's houses and go over their records together to assure quality control.
Transporting large numbers of Mesocyclops could be a problem because cyclopoids quickly exhaust their food supply when crowded in a small quantity of water. Then they eat each other. An easy solution came from the fact that these copepods can survive for months suspended on damp foam rubber, where they cannot move to eat each other. Foam rubber cubes are stacked in small plastic containers for mailing to public health offices throughout Vietnam. The copepods are introduced to a water storage container by dropping a foam rubber cube with 50 copepods into the container.
Most communities in the program have repeated the scenario at Phanboi, with Aedes aegypti declining to zero within 12-18 months after Mesocyclops introduction. The few exceptions have been urban communities, where Aedes aegypti has declined but not disappeared, the reason being incomplete coverage of the houses by local collaborators. It is sometimes necessary to recruit collaborators of unproved reliability in urban areas that lack ongoing house-to-house health programs. While most new collaborators do a good job, some do not, and their task can be complicated by lower social cohesion in cities. This year the program will face its greatest challenge as it extends to southern Vietnam, whose tropical climate is ideal for Aedes aegypti and dengue transmission throughout the year. New kinds of containers will be encountered as the program expands. For example, ant traps are a major breeding habitat in one area, and empty fish sauce jars that collect rainwater are a problem in another area. [2005 update: Copepods have successfully eradicated Ae. aegypti when used in southern Vietnam. Nationwide, they have eradicated Ae. aegypti in villages and urban neighborhoods with a total population of nearly a million people.]
Vietnam reported 234,000 Dengue Hemorrhagic Fever cases in 1998, responsible for more deaths than any other infectious disease. In 1999 the government initiated a high-priority national dengue program with Mesocyclops in a leading role. The program has both emergency response and prevention. The government provides MacElisa kits to local health workers for rapid blood analysis of suspected dengue cases so an immediate emergency response can go into action wherever dengue is confirmed. Health teams use ultra-low-volume backpack sprayers to apply insecticide to every house in an outbreak neighborhood. In recent years the government has encouraged dengue outbreak neighborhoods to capture fish from local ponds to put in their water storage containers, but as the supply of Mesocyclops increases, Mesocyclops will be mailed to outbreak areas on foam rubber cubes for immediate distribution to every house.
The key to sustainable long-term Dengue Hemorrhagic Fever control is prevention, which in Vietnam’s national dengue program means applying the Phanboi model. Television publicity and school education programs are making Mesocyclops a household word. A Government Inquiry Telephone Line refers interested communities to health workers who can provide Mesocyclops and explain their use. With twelve million households in high-risk areas, the potential number of households to be served is enormous. The bottleneck is training health workers and local collaborators. Some provinces are now setting up their own Mesocyclops production and training centers.
Conclusions
What does the Dengue Hemorrhagic Fever story tell us? First of all, it shows how human activities create environmental conditions that determine whether a disease will flourish or disappear. International transportation created Dengue Hemorrhagic Fever by moving the four dengue serotypes around the world. Dengue disappears when people eliminate the opportunities for Aedes aegypti to breed in water-filled containers around their homes.
Second, the story tells us that local mosquito eradication is possible with ecological management. To aim for less than eradication is to invite failure. An ecological disease control strategy that integrates a variety of control methods is more effective than a strategy based exclusively on pesticides, and we can expect ecological methods to be sustainable. It is unlikely that mosquito larvae will evolve resistance to Mesocyclops.
The story demonstrates the level of effort necessary for success. The effort that prevails nearly everywhere in the world today does not meet that standard. Nor does it meet the standard of the yellow fever campaign that eradicated Aedes aegypti from much of Brazil sixty years ago, a campaign that owed its success to its intensity and its meticulous organization and management.
Finally and most important, the story demonstrates the central role of local community. Dengue Hemorrhagic Fever will be eliminated only through an intense and well-organized effort at the local level. The general lack of progress with dengue during the past thirty years is not unique. Social support systems in local communities have declined throughout the world as personal and public priorities have shifted in other directions. Numerous dimensions of human welfare that depend on strong local communities have declined correspondingly. Ecologically sustainable development, including sustainable control of mosquito-transmitted diseases, will become a reality only when and where local communities are truly functional. While responsibility for a strong and effective local community must reside primarily with local citizens, encouragement and assistance from national governments can be decisive.
Can other countries use Mesocyclops as successfully as Vietnam? The prospects are particularly promising in Southeast Asia, where Dengue Hemorrhagic Fever is a major health problem, public concern is high, and most Aedes aegypti breeding habitats are similar to the water storage containers that have proved ideal for Mesocyclops in Vietnam. Public motivation is not so strong outside Southeast Asia, and some of the breeding habitats are not so ideal for Mesocyclops. While dengue control in other areas will often require substantially more than Mesocyclops and container recycling, Mesocyclops can eliminate Aedes aegypti production from at least some kinds of containers almost everywhere that dengue is a problem. Marco Suarez, for example, is using Mesocyclops longisetus in small storm-drain catch basins in Colombia.
The mechanics of production and distribution are not an obstacle to extending Mesocyclops to other countries. Production is inexpensive, and shipment to local distributors is easy. While production and distribution in Vietnam is by national, provincial, and local government, distribution in other countries could use any combination of government, NGOs, and the private sector that works under local conditions. The key to successful use of Mesocyclops is community organization. It is straightforward enough to put copepods in containers and re-stock the containers whenever copepods are lost, but it is essential to make sure that everyone does it. Success can proceed neighborhood by neighborhood. One hundred houses that work together can free themselves of Aedes aegypti even if houses in the surrounding area do nothing.
The most promising strategy is to distribute Mesocyclops where local networks provide the greatest prospects for success. Vietnam has the advantage that most of its dengue is in rural areas where community organization is strongest and house-to-house health programs are already functioning well. Fortunately, thousands of communities in other countries also have house-to-house networks of one sort or another for primary health care, family planning, paramedical malaria treatment, agricultural extension, religious charity, small business support, etc. These same networks could serve as vehicles for distributing Mesocyclops and ensuring their proper use on a community scale. Even private marketing networks, which so effectively distribute insecticide spray cans and mosquito coils, could have a role if rewards based on community use are built into the incentive system. With each success, the demonstration effect should stimulate more communities to organize so they can use Mesocyclops successfully.
References
- M.D. Brown, B.H. Kay, and J.K. Hendrix. 1991. Evaluation of Australian Mesocyclops (Copepoda: Cyclopoida) for mosquito control. J. Med. Entomol. 28: 618-623.
- S.R. Christophers. 1960. Aedes Aegypti (L.). The Yellow Fever Mosquito. Its Life History, Bionomics and Structure. Cambridge Univ. Press, 721 pages.
- D. Focks, D.G. Haile, E. Daniels, and G. Mount. 1993. Dynamic life table model for Aedes aegypti (Diptera: Culicideae): analysis of the literature and model development. J. Med. Entomol. 30: 1003-1017.
- C.D. Jennings, B. Phommasack, B. Sourignadeth, and B.H. Kay. 1995. Aedes aegypti control in the Lao People’s Democratic Republic, with reference to copepods. Am J. Trop. Med. Hyg. 53: 324-330.
- S.B. Halstead. 1997. Epidemiology of dengue and dengue hemorrhagic fever. In D.J. Gubler and G. Kuno (eds.), Dengue and Dengue Hemorrhagic Fever, p. 23-44. CAB International, New York.
- S.B. Halstead. 1998. Dengue and dengue hemorrhagic fever. In R.D. Feigin and J.D. Cherry (eds.), Textbook of Pediatric Infectious Diseases, p. 1984-1993. W.B. Sanders Co, Philadelphia.
- G.G. Marten. 1984. Impact of the copepod Mesocyclops leuckarti pilosa and the green alga Kirchneriella irregularis upon larval Aedes albopictus (Diptera: Culicidae). Bull. Soc. Vector Ecol. 9: 1-5.
- G.G. Marten, R. Astaeza, M.F. Suárez, C. Monje, and J.W. Reid. 1989. Natural control of larval Anopheles albimanus (Diptera: Culicidae) by the predator Mesocyclops (Copepoda: Cyclopoida). J. Med. Entomol. 26: 624-627.
- G.G. Marten. 1990a. Evaluation of cyclopoid copepods for Aedes albopictus control in tires. J. Am. Mosq. Control Assoc. 6: 681-688.
- G.G. Marten. 1990b. Elimination of Aedes albopictus from tire piles by introducing Macrocyclops albidus (Copepoda, Cyclopidae). J. Am. Mosq. Control Assoc. 6: 689-693.
- G.G. Marten, M. Cush, E. Fernández, G. Borjas, and H. Portillo. 1992. Mesocyclops longisetus and other forms of biological control for Aedes aegypti larvae in the Integrated Dengue Control Project, El Progreso, Honduras. In S.B. Halstead and H. Gomez-Dantes (eds.), Dengue--a worldwide problem, a common strategy, Proc. International Conference on Dengue and Aedes aegypti Community-based Control, p. 133-137. Mexican Ministry of Health and Rockefeller Foundation, Mexico.
- G.G. Marten, W.Y. Che, and E.S. Bordes. 1993. Compatibility of cyclopoid copepods with mosquito insecticides. J. Am. Mosq. Control Assoc. 9: 150-154.
- G.G. Marten, E.S. Bordes, and M. Nguyen. 1994a. Use of cyclopoid copepods for mosquito control. Hydrobiologia 292/293:491-496.
- G.G. Marten, G. Borjas, M. Cush, E. Fernández, and J.W. Reid. 1994b. Control of larval Ae. aegypti (Diptera: Culicidae) by cyclopoid copepods in peridomestic breeding containers. J. Med. Entomol. 31: 36-44.
- G.G. Marten, M. Nguyen, G. Thompson, and E.S. Bordes. 1997. Copepod production and application for mosquito control. New Orleans Mosquito Control Board, New Orleans, Louisiana, 43 pages.
- V.S. Nam, N.T. Yen, B.H. Kay, G.G. Marten, and J.W. Reid. 1998. Eradication of Aedes aegypti from a village in Vietnam, using copepods and community participation. Am. J. Trop. Med. Hyg. 59:657-660.
- F. Riviere and R. Thirel. 1981. La predation du copeopods Mesocyclops leuckarti pilosa sur les larves de Aedes (Stegomyia) aegypti et Ae. (St.) polynesiensis essais preliminaires d’utilization comme de lutte biologique. Entomophaga 26: 427-439.
- F. Riviere, B.H. Kay, J.M. Klein, and Y. Sechan. 1987. Mesocyclops aspericornis (Copepoda) and Bacillus thuringiensis var. israelensis for the biological control of Aedes and Culex vectors (Diptera: Culicidae) breeding in crab holes, tree holes, and artificial containers. J. Med. Entomol. 24: 425-430.
- B.M. Russell, L.E. Muir, P. Weinstein, and B.H. Kay. 1996. Surveillance of the mosquito Aedes aegypti and its biocontrol with the copepod Mesocyclops aspericornis in Australian wells and gold mines. Med. Vet. Entomol. 10: 155-160.
- F.L. Soper, D.B. Wilson, S. Lima, and W.S. Antunes. 1943. The organization of permanent nation-wide anti-Aedes aegypti measures in Brazil. The Rockefeller Foundation, New York, 137 pages.
- M.F. Suarez, D. Ayala, M.J. Nelson, and J.W. Reid. 1984. Hallazgo de Mesocyclops aspericornis (Daday) (Copepoda: Cyclopoidae) depredador de larvas de Aedes aegypti en Anapoima-Colombia. Biomedica 4: 74-76.
- W. Vasconcelos, C. Sleigh, B.H. Kay, C.P. Cabral, D.B. Araujo, Z.M. Ribiero, P.H. Braga, and J.S. Cavalcante. 1992. Community use of copepods to control Aedes aegypti in Brazil. In S.B. Halstead and H. Gomez-Dantes (eds.), Dengue--a Worldwide Problem, a Common Strategy, Proc. International Conference on Dengue and Aedes aegypti Community-based Control, p. 139-144. Mexican Ministry of Health and Rockefeller Foundation, Mexico.